This page has been archived on the Web
Information identified as archived is provided for reference, research or recordkeeping purposes. It is not subject to the Government of Canada Web Standards and has not been altered or updated since it was archived. Please contact us to request a format other than those available.
Reference Method for Source Testing: Measurement of Releases of Selected Semi-volatile Organic Compounds from Stationary Sources
Table of Contents
Canadian Cataloguing in Publication Data
Main entry under title:
Reference method for source testing : measurement of releases of selected semi-volatile organic compounds from stationary sources
(Report ; EPS 1/RM/2)
Text in English and French with French text on inverted pages.
Title on added t.p.: Méthode de référence en vue d’essais aux sources, dosage des composés organiques semi-volatils dans les émissions de sources fixes.
Issued by Environment Canada.
Includes bibliographical references.
ISBN 0-662-56823-0
DSS cat. no. En49-24/1-2
- Hazardous wastes -- Canada -- Incineration.
- Organic compounds -- Waste disposal.
- Air -- Pollution -- Measurement.
- Canada. Pollution Measurement Division.
- Canada. Environment Canada.
- Series: Report (Canada. Environment Canada); EPS l/RM/2.
© Minister of Supply and Services Canada 1989
Catalogue No. En 49-21/1-2E
ISBN 0-662-56823-0
Beauregard Printers Limited
Reader's Comments
Inquiries pertaining to the use of this reference method should be directed to:
Pollution Measurement Division
Technology Development and Technical Services Branch
Conservation and Protection
Environment Canada
River Road Environmental Technology Centre
Ottawa, Ontario
K1A 0H3
Additional copies of this report are available from:
Technology Transfer and Training Division
Technology Development and Technical Services Branch
Conservation and Protection
Environment Canada
Ottawa, Ontario
K1A 0H3
Foreword
The Environment Canada Reference Method (RM) described in this document is used to measure the releases of selected semi-volatile organic compounds from stationary sources. This method is used in conjunction with those described in Environment Canada Report EPS 1-AP-74-1, "Standard Reference Method for Source Testing: Measurement of Emissions of Particulates from Stationary Sources", as amended. The complexity of method procedures warrants that personnel performing them be trained and experienced.
Application of this RM for compliance testing requires strict adherence to the method in all respects. Deviation from the method may invalidate the test results. Any changes in equipment, reagents, materials, procedures, or calculations from those specified in the RM must be approved in writing by Environment Canada prior to testing. If deviations are made without prior approval, the validities of the tests shall be determined by Environment Canada on a case-by-case basis.
Note: Mention of trade names or commercial products does not constitute endorsement for use by Environment Canada.
Section 1: Applicability
This Environment Canada Reference Method (RM) is applicable to the determination of emissions of polychlorinated dibenzo-para-dioxins (PCDDs), polychlorinated dibenzofurans (PCDFs), polychlorinated biphenyls (PCBs), and other semi-volatile organic compounds (SVOCs) from stationary sources. Semi-volatile organics are defined as compounds with boiling points greater than 100°C. The testing procedures in Method A, B, C, D and F, contained in Environment Canada Report EPS 1-AP-74-1, "Reference Methods for Source Testing: Measurement of Emissions of Particulates from Stationary Sources" (as amended), are to be used in conjunction with the SVOC method:
- Method A - Determination of Sampling Site and Traverse Points
- Method B - Determination of Stack Gas Velocity and Volumetric Flow Rates
- Method C - Determination of Molecular Weight by Gas Analysis
- Method D - Determination of Moisture Content
- Method F - Calibration Procedure for S-Type Pitot Tube, Dry Gas Meter and Orifice Meter.
Section 2: Principle
An integrated stack gas sample is isokinetically withdrawn from a number of traverse points along the stack cross section. Semi-volatile organic compounds associated with particulate matter are collected in the front-half components of the sampling train. Semi-volatile organic compounds not collected by the high efficiency glass or quartz fibre filter are adsorbed on a porous, polymeric resin, Amberlite XAD-2.
Section 3: Apparatus
3.1 Sampling Train (Figure 1)
Figure 1: Semi-Volatile Organics Sampling Train
The following equipment is required:
- Nozzle
- A button-hook type nozzle with a sharp, tapered leading edge. The nozzle is usually made of 316 stainless steel or Incoloy 825, but quartz or equivalent material may be used when high temperature or corrosive gases are encountered. The minimum inside diameter of the nozzle shall be 4.76 mm (3/16 inch).
- Probe
- A borosilicate or quartz glass liner, encased in a stainless steel tube with a heating and temperature indicating system capable of maintaining the exit gas temperature at 120 ± 14°C (248 ± 25°F), or at such a temperature necessary to prevent condensation. Where length or strength limitations preclude the use of a glass liner, a seamless tubing made from an inert material such as Teflon may be used. A water-cooled probe should be used when very hot gases which could damage the nozzle/probe assembly are encountered.
- Pitot Tube
- A calibrated S-type (Stausscheibe) pitot tube mounted on the probe assembly.
- Stack Temperature Sensor
- A thermocouple, or other suitable temperature sensor, mounted on the probe assembly, capable of measuring the stack temperature to within 1.5% of the minimum absolute stack temperature. When high temperature gases are encountered, appropriate shielding and aspiration should be provided for the thermocouple so that radiation effects are avoided.
- Cyclone (Optional)
- A borosilicate miniature cyclone placed after the sampling probe and before the filter may be used to collect large particles. The cyclone prevents premature buildup of particulate matter on the filter medium and permits longer sampling periods. The cyclone is located inside the filter compartment and is kept at a temperature of 120 ± 14°C (248 ± 25°F).
- Filter Holder
- A borosilicate filter holder with an inert filter support. The filter support, which consists of a perforated disc, shall be constructed of Teflon to provide a seal against leakage at any point along the filter circumference. The filter holder is located inside the filter compartment and is maintained at a temperature of 120 ± 14°C (248 ± 25°F).
- Filter
- A high-efficiency glass or quartz fibre filter, which contains no organic binders and is capable of at least 99.95% efficiency for 0.3 μm dioctyl phthalate smoke particles as determined by ASTM Method D2986-71.
- Filter Compartment Heating System
- A heating system capable of maintaining the temperature in the filter holder compartment at 120 ± 14°C (248 ± 25°F), or at such a temperature necessary to prevent blinding of the filter. A thermocouple or other temperature sensor must be able to read the filter compartment temperature to within 3°C (5°F).
- Organic Sampling Module
- This unit consists of a gas condensing coil, a sorbent trap and a condensate knockout trap. The condenser must be able to condition the stack gas leaving the back-half of the filter holder at a temperature below 20°C (68°F). The sorbent trap shall be sized to contain 30-40 g of porous polymeric resin, Amberlite XAD-2. The sorbent trap should contain a glass well to accommodate a thermocouple to permit monitoring of the temperature of the gas sample entering the sorbent. The organic sampling module shall be oriented so that the condensate flows vertically, downward through the XAD-2 resin, into the condensate trap. The condensate trap shall be suitably sized and designed to prevent bubbling and carryover of condensate into the impingers.
A peristaltic or submersible pump shall be used to circulate the coolant through the XAD-2 trap and condenser. Ice water from the impinger bath may be used as the coolant.
All components of the organic module (condenser, XAD-2 trap and condensate trap) shall be fabricated of borosilicate glass. These components are not available commercially, but they may be custom fabricated from available laboratory glassware.
- Impingers
- Three Greenburg-Smith impingers are connected in series. The second and third impingers are modified by replacing the tips and impaction plates of the standard design with a 13 mm (0.5 inch) inside diameter glass tube extending to within 13 mm (0.5 inch) from the bottom of the impinger. The first impinger has the standard tip and impaction plate. The impingers are contained in an ice bath during sampling. The first impinger shall contain a known volume of ethylene glycol. The second shall remain empty and the third shall contain a known weight of silica gel.
- Vacuum Pump
- A leakproof vacuum pump capable of maintaining an isokinetic sampling rate while continuously withdrawing a portion of the stack gases through the samplingtrain is used. The pump intake vacuum is measured to within 13 mm mercury (Hg) (0.5 inch Hg) by a vacuum gauge which is attached to the vacuum line connecting the pump to the last impinger outlet. The sample flow rate is controlled with both coarse-and fine-flow control valves.
- Dry Gas Meter
- A calibrated dry gas meter with inlet and outlet temperature sensors, or one that is temperature-compensated. The sensors must be capable of measuring the temperatures to within 3°C (°F).
- Orifice
- A calibrated orifice connected to the outlet of the dry gas meter is required.
- Differential Pressure Gauges
- Differential pressure gauges such as an inclined manometer, or a device of equal sensitivity, which are capable of measuring the pitot tube velocity pressure and the pressure drop across the orifice to within 0.13 mm (0.005 inch) H2O on the 0 to 25 mm (0 to 1 inch) H2O scale, and readable to the nearest 1.3 mm (0.05 inch) H2O for ΔP values greater than 25 mm H2O. A more sensitive device shall be required when ΔP values are less than 1.3 mm H2O (0.05 inch H2O).
- Barometer
- A mercury, aneroid, or other barometer capable of measuring atmospheric pressure to within 2.5 mm Hg (0.1 inch Hg). Alternatively, the atmospheric pressure provided by the local weather office may be used with an adjustment for the elevation of the sampling site. Deduct 2.5 mm Hg (0.1 inch Hg) per 30.5 m (100 feet) of elevation increase or add 2.5 mm Hg (0.1 inch Hg) per 30.5 m (100 feet) of elevation decrease.
3.2 Sample Preparation and Recovery
- Probe Brush
- A nylon bristle brush of a length and diameter suitable for cleaning the probe. The brushes shall be cleaned prior to field use and shall have stainless steel or Teflon extensions.
- Wash Bottles
- Teflon wash bottles are necessary to dispense the recovery solvents. Polyethylene wash bottles shall not be used as the organic solvents stored in the bottle may extract organic contaminants.
- Sample Storage Containers
- Wide-mouthed amber glass bottles to store the hexane and acetone rinse. The screw caps shall be lined with Teflon lid liners. Both the amber glass bottles and the Teflon lid liners shall be cleaned. The amber glass bottles shall be proofed prior to field use. Both 500-mL and 1000-mL capacity bottles are required.
- Petri Dishes
- Glass petri dishes to store and transport the filters. The glass petri dishes shall be cleaned before field use.
- Teflon Lid Liners
- Teflon sheet to line the inside of the screw caps. The lid liners shall be cleaned prior to field use.
- Teflon Tape and Sleeves
- Teflon tape is necessary to seal the circumference of the filter holder and the glass ball joints. Both 13-mm (1/2-inch) and 25-mm (1-inch) wide tape is required. The Teflon sleeves are used to seal the tapered impinger joints.
- Glass Wool
- Glass or quartz wool to plug the unfritted end of the sorbent trap. The wool shall be cleaned prior to field use.
- Aluminum Foil
- Aluminium foil to wrap the loaded filter and to cover the open ends of the train glassware. The foil shall be cleaned before use.
- Miscellaneous
- Miscellaneous glassware such as graduated cylinders, glass plugs and caps and funnels. This glassware shall be cleaned prior to field use. Tweezers (stainless steel or Teflon) and disposable plastic gloves are also required. Do not use latex gloves or gloves with powdered inside surfaces.
Section 4: Reagents
All reagents used for organic testing shall be analyzed for organic contaminants prior to sampling. These reagents include the cleaning and rinsing solvents, High Purity Liquid Chromatography (HPLC) water, ethylene glycol and the XAD-2 resin.
- Solvents
- Solvents used for sampling shall be "Distilled-in-Glass" grade or "Pesticide Grade". These include methylene chloride (dichloromethane) hexane and acetone. Solvents may be screened by lot or batch number. Solvents from different batches will require a separate analysis.
- HPLC Water
- HPLC water for sample recovery is required.
- Ethylene Glycol
- Distilled-in-glass grade ethylene glycol shall be added to the first impinger. The glycol serves as a backup collection medium should organics break through the XAD-2 resin.
- Amberlite XAD-2 Resin
- Porous polymeric resin, XAD-2, is necessary to trap the gaseous semi-volatile organic compounds. The resin shall be cleaned and proofed before field use. Once cleaned, the XAD-2 should be stored in wide-mouthed amber glass jars with Teflon-lined caps and wrapped in aluminum foil. Resin not used within four weeks of preparation should be re-cleaned. The analytical procedure and acceptance criteria are described in the method specified in the Chemical Analysis Section. All XAD-2 traps (including the blank train and reagent blanks) must be loaded at the same time in a clean environment.
- Ice
- Crushed ice is required to maintain the impinger bath at a constant temperature.
- Silica Gel
- Indicating-type 6-16 mesh silica gel that has been dried at 180°C (356°F) for two hours is required.
Section 5: Procedures
5.1 Cleaning and Proofing
Before the field tests, all train glassware, amber storage bottles, Teflon lid liners, probe brushes, glass wool, petri dishes and aluminum foil must be cleaned as described in Table 1. The cleaning procedures also apply to miscellaneous items such as graduated cylinders, plugs and caps, funnels and tweezers. Other cleaning procedures are recognized, but the components must be proofed according to the procedures detailed in Table 1 and pass the acceptance criteria.
| Component | Cleaning | Proofing |
|---|---|---|
* Proofing not required for these components. Notes:
| ||
Glassware including: |
|
|
| Amber glass bottles |
|
|
| Teflon lid liners, Aluminum foil |
|
|
| Glass wool |
|
|
| Amberlite XAD-2 |
|
|
Caution: Handle all Solvents in a Well-Ventilated Area
The effectiveness of the cleaning procedures is verified by proofing selected components and reagents. Proofing ensures that the glassware, reagents and recovery solvents are free of pollutants prior to sampling. All components coming into contact with the stack gas or recovery samples must be cleaned, but it is not necessary to proof all the cleaned components. The proofing samples are analyzed as specified in Section 6, using High Resolution Gas Chromatography/Low Resolution Mass Spectography. The components and reagents that require proofing are as follows:
5.1.1 Train Glassware
Glassware cannot be re-used in the field with semi-volatile organic sampling. As a result, one complete set of pre-cleaned and proofed glassware must be allowed for each stack test. In addition, one complete set of glassware must also be allowed for the blank train. Assemble the complete sets of train glassware that will be needed and identify each set. Following the cleaning procedure described in Table 1, rinse each component from a train set three times each with hexane and acetone. Combine the rinses from each train into a pre-cleaned amber bottle with a pre-cleaned Teflon lid liner. Label the container. This sample constitutes the proof rinse for that train set. Repeat the above procedure for the other train sets and the blank train. Submit the proof rinses (one bottle per train) to the analytical lab for analysis.
5.1.2 Amber Glass Storage Bottles
Following the cleaning procedure, select three or four bottles from each box (48 bottles). Rinse these bottles three times each with hexane and acetone. Combine rinsings from each bottle in a pre-cleaned amber bottle with a pre-cleaned Teflon lid liner. Label the container and submit the proof rinse to the analytical laboratory. One proof rinse shall be submitted for each box of bottles.
5.1.3 Amberlite XAD-2 Resin
A 30 g aliquot of each cleaned batch is taken and analyzed. This procedure is described in greater detail in the analytical method specified in Section 6.
5.1.4 Hexane and Acetone
Submit a sample of each solvent in a pre-cleaned amber bottle with a pre-cleaned Teflon lid liner. Label each container, including the batch or lot number. Take an aliquot of each solvent to form a combined hexane/acetone proof rinse. Submit the combined rinse to the analytical lab for analysis.
5.1.5 Ethylene Glycol and HPLC Water
Submit a sample of each reagent in a pre-cleaned amber bottle with a pre-cleaned Teflon lid liner. Label each container and include the batch or lot number. Take an aliquot of each reagent to form a combined glycol/HPLC water proof rinse. Submit the combined rinse to the analytical lab for analysis.
5.2 Sample Collection
5.2.1 Preliminary
Select the sampling site and the minimum number of traverse points according to procedures described in Method A of Environment Canada Report EPS 1-AP-74-1, "Reference Method of Source Testing: Measurement of Emissions of Particulates from Stationary Sources" (as amended). Without previous knowledge of the stack variables, a preliminary test should be conducted to obtain the following data:
- velocity profile across the stack (Method B)
- stack temperature and pressure (Method B)
- stack gas molecular weight (Method C)
- stack gas moisture content (Method D)
Use the information to select the largest nozzle possible for isokinetic sampling. The recommended minimum nozzle size is 4.76 mm (3/16 inch) inside diameter. A nozzle size should be selected such that 3 to 4 m3 (105.9 - 141.3 ft3) of dry standard sample gas will be collected over an approximate four-hour sampling period.
Select a suitable probe length that will permit access to all the sampling points.
Select a total sampling time so that the sampling time per traverse point is equal to or greater than five minutes.
5.2.2 Sampling Train Preparation
Prepare the sampling train in a clean area to minimize contamination. Install the selected nozzle on the probe. Mark the probe to denote the location of each sampling point. Before preparing and assembling the train glassware, the components should be rinsed once each with hexane and acetone. Discard the rinses.
Carefully wrap the filter support with 25 mm (1-inch) Teflon tape along the circumference to provide a leak-free seal between both halves of the filter holder. Using a pair of tweezers, remove the filter from its petri dish, inspect for flaws and place on the perforated Teflon filter support. It is recommended that the filter assembly be "leak-checked" at 381 mm Hg (15 inches Hg) prior to assembly at the sampling site.
Add approximately 100 mL of ethylene glycol to the first impinger and about 200-300 g of silica gel to the third impinger. Weigh the condenser, XAD-2 trap, condensate trap and the three impingers to the nearest 0.5 g. Record each weight on the Moisture Analysis Data Sheet (Figure 2).
Figure 2: Moisture Analysis Data Sheet
During the assembly of the train, the joints must be sealed by wrapping the ball joints with 13-mm (0.5-inch) Teflon tape. Do not use vacuum grease to seal the joints. The configuration of the glassware will determine to what extent the train can be assembled in the field lab. From train assembly to extraction of the stack sample, the open glassware joints must be sealed with pre-cleaned glass plugs or caps or aluminum foil at all times.
At the sampling site, set up the train as shown in Figure 1. Connect all temperature sensors and pitot lines. Adjust the filter compartment and probe heating systems to maintain a temperature of 120 ± 14°C (248 ± 25°F). Connect the cooling system. Do not add the water or ice until the pre-test leak check is completed.
Once the filter compartment and probe have reached the necessary temperatures, conduct a leak check on the sampling train. This is done by plugging the nozzle inlet and pulling a vacuum of 381 mm Hg (15 inches Hg). A leakage rate of less than 0.57 l/min (0.02 ft3/min) or four percent of the estimated average sampling rate, whichever is less, is acceptable. Unacceptable leakage rates must be corrected. Record the leakage rate and the vacuum on the Organics Sampling Data Sheet (Figure 3). Place water and crushed ice in the impinger bath and turn on the coolant recirculating pump for the condenser coil and XAD-2 tube. During testing, the temperature of the XAD-2 must not exceed 20°C (68°F) for effective removal of the semi-volatile organic species. At all other times the XAD-2 must not be exposed to temperatures above 50°C (122°F) to avoid thermal degradation.
Figure 3: Organics Sampling Data Sheet
Click to enlarge
5.2.3 Sampling Train Operation
Verify the heating and cooling systems and check that the probe and pitot tube are properly aligned. Level and zero the manometer. Clean the access port to avoid extraneous pick up of deposited material. To begin sampling, point the nozzle directly into the gas stream at the first traverse point. Block off the opening between the probe assembly and the access ports. With a nomograph or a programmable calculator, determine the orifice setting for isokinetic sampling. Record the initial dry gas meter reading. Immediately start the vacuum pump and adjust the sampling flow rate to isokinetic conditions. Sample for at least five minutes at each traverse point, the sampling time being the same for every point. Traverse the stack cross section and maintain isokinetic sampling (±10%) throughout the test. Record instrumentation readings on the Organics Sampling Data Sheet (Figure 3) every five minutes, or at regular intervals that are consistent with the sampling duration established for each point, whichever is less. To simplify recording in the field, values may be entered in units for which the sampling equipment is designed. These values may then be converted (if necessary) to the metric units specified in the equations where they are used. Readings must also be taken before and after a "leak-check" and when sampling is halted. Record all sampling interruptions.
During sampling, verify the heating and cooling systems. The temperature of the filter enclosure and probe must be 120 ± 14°C (248 ± 25°F). The XAD-2 temperature must be below 20°C. Verify the alignment of the probe and pitot tube to the gas stream. Check the level and zero of the manometer and adjust if necessary. Verify that the condensate volume is increasing as the run progresses. If the sampling system vacuum becomes so high that it is difficult to maintain isokinetic sampling the filter holder and/or sorbent tube must be replaced. If this occurs, a "leak-check" must be conducted before and after the replacement of the above components.
When it is necessary to halt sampling temporarily either to dismantle the sampling train during port change-over or to change a train component, turn off the pump and immediately withdraw the probe from the stack. Conduct a mandatory "leak-check" on the sampling train by plugging the nozzle and pulling a vacuum equal to or greater than the maximum value observed during sampling. Record the actual leakage rate. If the leakage rate exceeds 0.57 l/min (0.02 ft3/min) or four percent of the sampling flowrate, whichever is less, consult Environment Canada regarding the validity of the test. If the leakage rate is acceptable, proceed with dismantling the sampling train or changing the train component. Before continuing the test, conduct a "leak-check" on the assembled train by following the pre-test "leak-check" procedures used during sampling train preparation.
When the test is completed, conduct a mandatory post-test check on the sampling train by plugging the nozzle and pulling a vacuum equal to or greater than the maximum value observed during sampling. Record the actual leakage rate which must be less than either 0.57 l/min (0.02 ft3/min) or four percent of the sampling flow rate during the test, whichever is less. If the leakage rates are acceptable, proceed with the recovery of the samples.
When the test is finished, disconnect the probe and set it aside to cool. Seal both ends and take care not to lose any material in the probe. Disconnect the filter (and cyclone, if used) and seal both ends with pre-cleaned glass plugs or caps, or aluminum foil. Disconnect the XAD-2 and condenser. Seal both ends. Seal the ends of the impinger train. When transporting the sampling train components to the on-site lab, take care to minimize the possibility of sample loss or contamination. Samples must be recovered in a clean area.
5.2.3 Blank Train
One blank train must be submitted for every group of three sample trains. The blank train is handled in the same manner as the loaded train except that no stack gases are drawn through the train. However, a volume of ambient air equal to that drawn during the pre- and post-traverse "leak-checks" must be drawn through the blank train. Hence, on the day that the blank train will be submitted, it is necessary to record all volumes drawn into the sampling train during all "leak-checks". It is not necessary to heat the filter components of the blank train during sampling or to conduct "leak-checks" on the blank train. The sample recovery procedures for the loaded trains are also applicable to the blank train.
5.3 Sample Recovery
5.3.1 Nozzle, Probe, Cyclone and Front-Half of the Filter Holder
Wipe exterior surfaces of the nozzle and probe to remove excess particulate matter. Quantitatively recover the particulate matter and condensibles from the nozzle, probe, cyclone (if used), by-pass connector (if cyclone is not used) and the front-half of the filter holder by washing (with brush) these components with hexane and acetone. Wash and brush each component three times with hexane and acetone. Finally, rinse each component three times each with hexane and acetone. Store all rinses in a wide mouth amber glass bottle (pre-cleaned, with a pre-cleaned Teflon lid liner). Mark the liquid level on the outside of the bottle and label the container. Place the labelled container in a sealed clear plastic bag.
Note: The extension rod (stainless steel or Teflon) of the probe brush used to wash the probe liner shall be wiped with the recovery solvents prior to sample recovery.
5.3.2 Filter
Carefully remove the exposed filter from the filter holder and place on precleaned aluminum foil with exposed side up. Carefully transfer any loose particulate matter or filter fibres adhering to the support of the filter holder with a dry nylon bristle brush or sharp blade. Fold the filter in half and crimp the foil to close the edges. Place in a pre-cleaned glass petri dish. Label the petri dish and seal with 25-mm (one inch) wide Teflon tape around the circumference. The petri dish may also be sealed with aluminum foil.
5.3.3 Back-half of Filter Holder and Condenser
Drain all the cooling water from the condenser and wipe the outside of the condenser to remove excess water. Weigh the condenser and record the weight on the Moisture Analysis Data Sheet. Soak each of these components five minutes each with hexane and acetone. Rinse each component three times each with hexane and acetone. Store the soak and rinses in a wide-mouth amber bottle (pre-cleaned, with a pre-cleaned Teflon lid liner). Mark the liquid level on the outside of the bottle and label the container. Place the labelled container in a sealed clear plastic bag.
5.3.4 XAD-2 Tube
Remove the aluminum foil on the tube and drain all the cooling water. Wipe the outside of the tube to remove excess moisture. Weigh the XAD-2 tube and record the weight on the Moisture Analysis Data Sheet. Seal both ends of the XAD-2 tube with pre-cleaned glass caps or plugs. Wrap the whole tube with aluminum foil and label the container.
5.3.5 Condensate Trap and Ethylene Glycol Impinger
Wipe the outside of these components to remove excess moisture. Weigh each component and record the weights on the Moisture Analysis Data Sheet. Empty each container into a pre-cleaned amber glass bottle with a pre-cleaned Teflon lid liner. Rinse each component three times each with HPLC water into the same container. Mark the liquid level on the outside of the bottle and label the container. Place the labelled container in a sealed clear plastic bag.
5.3.6 Second and Third Impingers
Wipe the excess moisture from the outside of these impingers and weigh each impinger. Record each weight on the Moisture Analysis Data Sheet.
5.3.7 All Back-Half Glassware excluding XAD-2 Tube
Rinse each component including connectors three times each with hexane and acetone into a pre-cleaned glass amber bottle with a pre-cleaned Teflon lid liner. Mark the liquid level on the outside of the bottle and label the container. Place the labelled container in a sealed clear plastic bag. The sample recovery procedures for the front- and back-half components are illustrated in Figure 4.
Figure 4: Semi-Volatile Organics Recovery Procedure
5.3.8 Sample Handling
Following the recovery of the train components, all samples must be kept cool (approximately 4°C). They may be stored in a refrigerator or on ice in an insulated chest during field storage and transport to the analytical laboratory.
Before transporting the insulated chest, it is critical to ensure that the containers are well-packed to minimize sample loss. Many materials are available to protect the glass container against shock. Extra care should be taken when packing the samples.
Section 6: Chemical Analysis
The amount of PCBs, PCDDs and PCDFs in the test samples shall be determined by the Chemical Analysis procedures found in the Environment Canada document, "A Method for the Analysis of Polychlorinated Dibenzo-para-Dioxins (PCDDs), Polychlorinated Dibenzofurans (PCDFs), and Polychlorinated Biphenyls (PCBs) in samples from the Incineration of PCB Waste", Chemistry Division, Conservation and Protection, EPS-1/RM/3, 1989. The analytical results are to be used in Equation 10, Equation 11 and Figure 5.
Section 7: Calculations
- 7.1 Equations
- 7.2 Nomenclature
To simplify recording during a test, field data may be entered in the units for which the sampling equipment is designed. These values must be converted, if necessary, to the metric units specified in the applicable equations.
7.1 Equations (see Section 7.2 for Nomenclature)
7.1.1 Dry Gas Meter Volume
The total sample volume measured by the dry gas meter is corrected to reference temperature and pressure conditions (25°C and 101.3 kPa) using Equation 1. For a temperature-compensated dry gas meter (Tm)avg should be substituted by the temperature specified by the manufacturer.
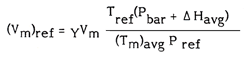
7.1.2 Volume of Water Vapour
The volume of water vapour in the sack gas sample expressed at reference conditions (25°C and 101.3 kPa) is calculated by using Equation 2.

7.1.3 Moisture Content
The volumetric fraction of water vapour in the stack gas expressed at reference conditions (25°C and 101.3 kPa) is calculated using Equation 3.

For a saturated or suspersaturated stack, use a psychrometric chart to determine Bwo.
7.1.4 Absolute Stack Gas Pressure
The absolute stack gas pressure is calculated using Equation 4.
![]()
7.1.5 Stack Gas Molecular Weight
The stack gas molecular weight on a wet basis is calculated using Equation 5.
![]()
7.1.6 Stack Gas Velocity
The stack gas velocity measured at each traverse point is calculated using Equation 6.

The average stack gas velocity ((Us)avg) and stack gas temperature ((Ts)avg) for the test run are determined by averaging the velocities and temperatures respectively measured at the traverse points.
7.1.7 Volumetric Stack Gas Flow Rate
The average volumetric stack gas flow rate expressed on a dry basis and at reference conditions (25°C and 101.3 kPa) is calculated using Equation 7.

7.1.8 Isokinetic Variation
The isokinetic variation for each traverse points is calculated using Equation 8.

A test shall be considered valid when no more than 10% of the isokinetic values fall outside the range 90% to 110% and the average for all the traverse points Iavg falls within the range 90% to 110%.
7.1.9 Correction Factor
The pollutant concentrations expressed in this method must be corrected to a basis of 11% O2. The correction factor to express concentrations at 11% O2 is calculated using Equation 9.

7.1.10 Polychlorinated Biphenyl Emission Rates
The emission rate of polychlorinated biphenyls is calculated using Equation 10.

7.1.11 Concentrations of 2,3,7,8-TCDD Equivalent PCDDs and PCDFs
The concentration of 2,3,7,8-TCDD equivalent PCDDs and PCDFs corrected to 11% O2 is calculated using Equation 11. The weight of 2,3,7,8-TCDD equivalent PCDDs and PCDFs is determined from Figure 5.

Figure 5: Calculation of 2,3,7,8-T4CDD Equivalent PCDDs and PCDFs
7.2 Nomenclature
- As
- inside cross-sectional area of stack or duct, m2
- Bwo
- volumetric fraction of water vapour in the stack gas, dimensionless
- C2,3,7,8-T4CDD'
- concentration of 2,3,7,8-T4CDD equivalent PCDDs and PCDFs on a dry basis at reference temperature and pressure conditions corrected to 11% O2, ng/m3 at 11% O2
- CP
- S-type pitot tube coefficient, dimensionless
- ERPCBs
- emission rate of polychlorinated biphenyls, μg/h
- ΔHavg
- average pressure drop across orifice meter, kPa
- ΔH
- pressure drop across orifice meter for each traverse point, kPa
- Iavg
- average isokineticity for the test, (%)
- I
- isokineticity i.e., the ratio of the sampling velocity through the nozzle to the velocity of the undisturbed gas stream at each traverse point, dimensionless, (%)
- j
- traverse point number, dimensionless
- Md
- molecular weight of stack gases on a dry basis, kg/kmol
- Ms
- molecular weight of stack gases on a wet basis, kg/kmol
- MH2O
- molecular weight of water, 18 kg/kmol
- Nd
- inside diameter of the sampling nozzle, mm
- f11% O2
- correction factor to convert concentrations at dry and reference temperature and presure conditions to 11% O2, dimensionless
- %O2
- concentration of oxygen in the stack gas (dry basis), % by volume
- Ps
- absolute stack gas pressure, kPa
- Pbar
- barometric pressure at the sampling site, kPa
- ΔP
- pitot-tube velocity pressure reading at each traverse point, kPa
- Pref
- reference pressure, 101.3 kPa
- ΔPs
- static pressure of the stack gas, kPa
- Qs
- volumetric stack gas flow rate on a dry basis at reference temperature and reference pressure conditions, m3/h
- R
- universal gas constant, 8.31 kPa m3(kmol·K)-1
- t
- sampling duration for each traverse point, min
- (Ts)avg
- average of the absolute stack gas temperatures, K
- Ts
- absolute stack gas temperature at each traverse point, K
- (Tm)avg
- average of the absolute dry gas meter temperatures, K
- Tmi
- absolute temperature at the dry gas meter inlet for each traverse point, K
- Tmo
- absolute temperature at the dry gas meter outlet for each traverse point, K
- Tref
- reference temperature, 298 K
- (Us)avg
- average stack gas velocity, m/s
- Us
- stack gas velocity measured at each traverse point, m/s
- Vm
- volume of stack gas sample at dry gas meter conditions, m3
- (Vm)j
- volume of stack gas sample at dry gas meter conditions for each traverse point, m3
- (Vm)ref
- volume of stack gas sample at reference conditions, m3
- As
- volume of water vapour in the stack gas sample at reference conditions, m3
- WH2O
- weight of water vapour condensed in the impingers, g
- WPCBs
- weight of polychlorinated biphenyls collected in the semi-volatile organics sampling train, mg
- W2,3,7,8-T4CDD'
- weight of 2,3,7,8-TCDD equivalent PCDDs and PCDFs collected in the semi-volatile organics sampling train, ng
- γ
- dry gas meter calibration factor (ratio of the wet test meter volume to the dry test meter volume) dimensionless
- 128.95
- proportionality constant (m/s)(K-1·kg/kmol)½
References
- Environment Canada, "Standard Reference Method for Source Testing: Measurement of Emissions of Particulates from Stationary Sources", Report EPS 1-AP-74-1, February 1974 (as amended).
- Bursey, J., M. Hartman, J. Homolya, R. McAllister, J. McGaughey, D. Wagoner. "Laboratory and Field Evaluation of the Semi-VOST Method, Volume II: Appendices", EPA/600/4-85/0756 NTIS No. PB 86-123569, November 1985.
Lists of Tables and Figures
List of Tables
- Table 1: Cleaning and Proofing Procedures
List of Figures
- Figure 1: Semi-Volatile Organics Sampling Train
- Figure 2: Moisture Analysis Data Sheet
- Figure 3: Organics Sampling Data Sheet
- Figure 4: Semi-Volatile Organics Recovery Procedure
- Figure 5: Calculation of 2,3,7,8-T4CDD Equivalent PCDDs and PCDFs
Figure 1
In Figure 1, a nozzle which is connected to a probe is placed in a stack. The sample flows through the nozzle and probe into a hot box where it is connected to a cyclone and then to the filter. The sample then passes through the filter into a condenser, where the organics are condensed and passed through a XAD resin. The XAD tube is then connected to a condensate trap where moisture is condensed and condensate is collected. Next the trap is connected to 3 impingers sitting in an ice bath. The impingers are connected to an umbilical line that connects to a pump with has fine and coarse valves and then to a console with a dry gas meter, orifice and manometer where the volume collected is read and recorded. The S type pitot tube is connected to a probe which is connected to a pitot manometer to measure the velocity.
Figure 2
Figure 2 is used to determine the mass of moisture. Record the following data before the run:
- Plant,
- Location,
- Run number,
- Date,
- Run conducted by.
The table is divided into 6 components:
- Condenser,
- Resin Trap,
- Condensate Trap,
- First Impinger,
- Second Impinger,
- Third Impinger.
Each component is weighed before the run (initial reading) and weighed after the run (final reading). The initial is subtracted from the final to produce a gain for each component. The gains from all the components are added together to calculate the mass of moisture collected. (note: All readings should be in grams)
Figure 3
Figure 3 is a data sheet used to record all data during a sample run. Record the following data before the run:
- Plant,
- Location,
- Run number,
- Date,
- Stack diameter,
- Probe length,
- Probe type,
- Filter media,
- Leak Check (CFM, inches mercury),
- Barometric Pressure,
- Nozzle diameter,
- Pitot coefficient,
- Moisture content,
- Dry molecular weight,
- Orifice meter coefficient,
- Dry test meter coefficient,
- Stack static pressure,
- Name of who's conducting the stack test.
During the run, the following information needs to be recorded:
- Point number,
- Time,
- Stack gas temperature,
- Velocity pressure,
- Orifice pressure,
- Gas meter volume reading,
- Gas meter temperature for both the inlet and outlet,
- Filter box temperature,
- Probe temperature,
- XAD-2 Inlet temperature,
- Pump vacuum.
Figure 4
This figure deals with a sketch of the glassware train and a table detailing the recovery procedure for each numbered piece of glassware. In container 1, wash and brush 3 times with Hexane the nozzle, probe liner, cyclone, and front half of the filter holder. Then wash and brush 3 times with Acetone. Into the same container rinse 3 times each part with Hexane and Acetone. In container 2, which is a Petri dish, carefully remove the filter from the filter holder. Place it on pre-cleaned foil. Fold in half. Place in a pre-cleaned glass Petri dish. For container 3, soak the back half of the filter holder and the condenser with Hexane for 5 minutes. Pour the Hexane into the container and then repeat with Acetone. Into the same container rinse 3 times each part with hexane and acetone. Container 4 is the actual XAD tube. Cap both ends and wrap with Aluminum foil. In container 5, empty contents from the condensate trap and the first impinger. Rinse each 3 times with HPLC water and pour into container. In container 6, rinse the following 3 times with Hexane. The back half of the filter holder, condenser, condensate trap, straight tube, all three impingers and connectors. Repeat 3 times with Acetone. Mark liquid levels on all bottles. All sample containers are pre-cleaned amber glass bottles with pre-cleaned Teflon lid liners.
Figure 5
This figure deals with a table to calculate the total mass of PCDDs and PCDFs. The table is divided into 4 sections:
- Congener,
- Train catch,
- Toxicity factor,
- Equivalent train catch.
Calculate each PCDD as follows:
- For 2,3,7,8-T4CDD, train catch multiplied by 1.0 toxicity factor equals equivalent train catch.
- For 1,2,3,7,8-P5CDD, train catch times 0.5.
- For 1,2,3,4,7,8-H6CDD, train catch times 0.1.
- For 1,2,3,6,7,8-H6CDD, train catch times 0.1.
- For 1,2,3,7,8,9-H6CDD, train catch times 0.1.
- For 1,2,3,4,6,7,8-H7CDD, train catch times 0.01.
- For O8CDD, train catch times 0.001.
Calculate each PCDF as follows:
- For 2,3,7,8-T4CDF, train catch multiplied by 0.1 toxicity factor equals equivalent train catch.
- For 1,2,3,7,8-P5CDF, train catch times 0.05.
- For 2,3,4,7,8-P5CDF, train catch times 0.5.
- For 1,2,3,4,7,8-H6CDF, train catch times 0.1.
- For 1,2,3,6,7,8-H6CDF, train catch times 0.1.
- For 1,2,3,7,8,9-H6CDF, train catch times 0.1.
- For 2,3,4,6,7,8-H6CDF, train catch times 0.1.
- For 1,2,3,4,6,7,8-H7CDF, train catch times 0.01.
- For 1,2,3,4,7,8,9-H7CDF, train catch times 0.01.
- For O8CDF, train catch times 0.001.
Add all equivalent train catch for each congener to calculate the weight of 2,3,7,8-T4CDD.
Equation 1
Add the barometric pressure in kPa to the average pressure drop across orifice meter also in kPa. Multiply this by the reference temperature, 298 K. Divide this value with the average of the absolute dry gas meter temperature in K multiplied by the reference pressure, 101.3 kPa. Multiply this new value with the volume of stack gas sample at dry gas meter conditions, m3 and the dry gas meter calibration factor, dimensionless.
Equation 2
Multiply the weight of water vapour condensed in the impingers, g, with ten to the negative third power, the universal gas constant, 8.31 kPa m3/kmol K, and the reference temperature. Divide this value with molecular weight of water, 18 kg/mol multiplied with reference pressure, 101.3 kPa.
Equation 3
Divide the volume of water vapour in the stack gas sample at reference conditions, m3, with the sum of volume of water vapour in the stack gas sample at reference conditions, m3, added to the volume of stack gas sample at reference conditions, m3. Or simply, use previous calculated values. Volume of water vapour divided by volume of water vapour added to dry gas meter volume.
Equation 4
Add the barometric pressure at the sampling site, kPa to the static pressure of the stack gas, kPa.
Equation 5
Subtract the volumetric fraction of water vapour in the stack gas, dimensionless, from 1 and multiply it by the molecular weight of stack gases on a dry basis, kg/kmol. Add this value to 18 multiplied by the volumetric fraction of water vapour in the stack gas, dimensionless.
Equation 6
Multiply the reference pressure, 101.3 kPa, with the absolute stack gas temperature at each traverse point, K. Divide this value with the absolute stack gas pressure, kPa, multiplied to molecular weight of stack gases on a wet basis, kg/mol. Take the square root of this value. Then multiply this with the number 128.95 and the S-type pitot tube coefficient, dimensionless.
Equation 7
Multiply the reference temperature, 298 K, with the absolute stack gas pressure, kPa. Divide this value with the average of the absolute stack gas temperature, K, multiplied to the reference pressure, 101.3kPa. Multiply this value with the volumetric fraction of water vapour in the stack gas, dimensionless, subtracted from 1. Multiply this value with the inside cross-sectional areas of stack or duct, m2, the average stack gas velocity, m/s, and 3600.
Equation 8
This equation is divided into 2 parts. For the first part, add the barometric pressure at the sampling site, kPa, to the pressure drop across orifice meter for each traverse point, kPa. Multiply this value with the absolute stack gas temperature at each traverse point, K. Multiply this value by 1 divided by 1 minus the volumetric fraction of water vapour in the stack gas, dimensionless. Multiply this value by the volume of stack gas sample at dry gas meter conditions for each traverse point, m3, divided by sampling duration for each traverse point, min. Multiply this final value with the dry gas meter calibration factor (ratio of the wet test meter volume to the dry test meter volume), dimensionless. For the second part add the absolute temperature at the dry gas meter inlet and outlet together for each traverse point, K, and divide by 2. Multiply this by 4.71 times ten to the negative fifth power, then multiply the inside diameter of the sampling nozzle squared, mm. Multiply this value with the absolute stack gas pressure, kPa and then the stack gas velocity measured at each traverse point, m/s. Take the value from the first part and divide by the second part. Multiply this final value by 100.
Equation 9
9.9 divided by 20.9 minus percent O2.
Equation 10
Multiply the weight of polychlorinated biphenyls collected in the semi-volatile organics sampling train, mg, with the volumetric stack gas flow rate on a dry basis at reference temperature and reference pressure conditions, m3/h. Divide this value with the volume of stack gas sample at reference conditions, m3.
Equation 11
Multiply the weight of 2,3,7,8-TCDD equivalent PCDDs and PCDFs collected in the semi-volatile organics sampling train, ng, to the correction factor to convert concentration at dry and reference temperature and pressure conditions to 11% O2, dimensionless. Divide this value with the volume of stack gas sample at reference conditions, m3.
- Date modified: