This page has been archived on the Web
Information identified as archived is provided for reference, research or recordkeeping purposes. It is not subject to the Government of Canada Web Standards and has not been altered or updated since it was archived. Please contact us to request a format other than those available.
Proposed Risk Management Approach for
2-Propanone, reaction products with diphenylamine
(PREPOD)
Chemical Abstracts Service Registry Number (CAS RN)
68412-48-6
Environment Canada
Health Canada
September 2011
Table of Contents
- Issue
- Background
- Why We Need Action
- Current Uses and Industrial Sectors
- Presence in the Canadian Environment and Exposure Sources
- Overview of Existing Actions
- Considerations
- Proposed Objectives
- Proposed Risk Management
- Consultation Approach
- Next Steps / Proposed Timeline
- References
This proposed risk management approach document builds on the previously released risk management scope document for PREPOD, and outlines the proposed control actions for this substance. Stakeholders are invited to submit comments on the content of this proposed risk management approach or provide other information that would help to inform decision making. Following this consultation period, the Government of Canada will initiate the development of the specific risk management instrument(s) and/or regulation(s) where necessary. Comments received on the proposed risk management approach will be taken into consideration in developing the instrument(s) and/or regulation(s). Consultation will also take place as instrument(s) and/or regulation(s) are developed.
Summary of Risk Management[*]
- The Government of Canada plans to add PREPOD to the Virtual Elimination List of the Canadian Environmental Protection Act, 1999 (CEPA 1999).
- The Government of Canada plans to develop a control instrument under CEPA 1999 to address releases to the environment from the manufacturing and industrial use of PREPOD, as appropriate.
- The Government of Canada plans to implement Significant New Activity (SNAc) provisions under CEPA 1999 to PREPOD.
- The Government of Canada will work with stakeholders to further quantify sources of releases of PREPOD throughout its lifecycle and will develop risk management control actions under CEPA 1999 to address these releases as required.
- The Government of Canada will add PREPOD to the CMP monitoring and surveillance program to quantify levels of this substance that may be found in the environment.
Note: This summary is an abridged list of the instruments and tools proposed to risk manage this substance. Please see section 9.1 of this document for a complete explanation of risk management.
1. Issue
1.1 Categorization and the Challenge to Industry and Other Interested Stakeholders
The Canadian Environmental Protection Act, 1999 (CEPA 1999) (Canada 1999) requires the Minister of the Environment and the Minister of Health (the Ministers) to categorize substances on the Domestic Substances List (DSL). Categorization involves identifying those substances on the DSL that, in accordance with the criteria at section 73 of the Act, a) are considered to be persistent (P) or bioaccumulative (B), based on the criteria set out in the Persistence and Bioaccumulation Regulations (Canada 2000), and “inherently toxic” (iT) to humans or other organisms, or b) may present, to individuals in Canada, the greatest potential for exposure (GPE). In addition, the Act requires the Ministers to conduct screening assessments of substances that meet the categorization criteria. The assessment further determines whether the substance meets one or more of the criteria set out in section 64 of the Act[1].
In December 2006, the Challenge identified 193 chemical substances through categorization which became high priorities for assessment due to their hazardous properties and their potential to pose risks to human health and the environment. In February 2007, the Ministers began publishing, for industry and stakeholder comments, profiles of batches containing 12 to 19 high-priority substances. New batches are released for comments every three months.
Information-gathering authority in section 71 of CEPA 1999 is being used under the Challenge to gather specific information where it is required. The information that is collected through the Challenge is used to make informed decisions and appropriately manage any risks that may be associated with these substances.
The substance 2-Propanone, reaction products with diphenylamine, Chemical Abstracts Service Registry Number (CAS RN)[2] 68412-48-6, referred to throughout this document as “PREPOD”, is included in Batch 11 of the Challenge under the Chemicals Management Plan (Canada 2009).
1.2 Final Screening Assessment Report Conclusion for PREPOD
A notice summarizing the scientific considerations of a final screening assessment report was published by Environment Canada and Health Canada in the Canada Gazette,Part I, for PREPOD on September 10, 2011 under subsection 77(6) of CEPA 1999. The final screening assessment report concluded that PREPOD is entering or may be entering the environment in a quantity or a concentration or under conditions that have or may have an immediate or long-term harmful effect on the environment or its biological diversity.
The final screening assessment report also concluded that many components of PREPOD meet the criteria for persistence and at least one component meets the criteria for bioaccumulation, as defined in the Persistence and Bioaccumulation Regulations made under CEPA 1999. The presence of PREPOD in the environment results primarily from human activity.
For further information on the final screening assessment report conclusion for PREPOD, refer to the final screening assessment report, available on the Chemical Substances in Batch 11 of the Challenge.
1.3 Proposed Measure
As a result of a screening assessment of a substance under section 74 of CEPA 1999, the substance may be found to meet one or more of the criteria under section 64 of CEPA 1999. The Ministers can propose to take no further action with respect to the substance, add the substance to the Priority Substances List (PSL) for further assessment, or recommend the addition of the substance to the List of Toxic Substances in Schedule 1 of the Act. Under certain circumstances, the Ministers must make a specific proposal to recommend the implementation of virtual elimination. In this case, the Ministers proposed to recommend the addition of PREPOD to the List of Toxic Substances in Schedule 1. As a result, the Ministers will develop a regulation or instrument respecting preventive or control actions to protect the environment from the potential effects of exposure to this substance.
In addition, the final screening assessment report concluded that
- PREPOD meets the criterion set out in paragraph 64(a)of CEPA 1999; and
- PREPOD is inherently toxic to non-human organisms, as determined by laboratory or other studies; and
- Many components of PREPOD are persistent and at least one component (Diisopropyldimethylacridan) is bioaccumulative in accordance with the Persistence and Bioaccumulation Regulations made under CEPA 1999; and
- the presence of PREPOD in the environment results primarily from human activity; and
- PREPOD is not a naturally occurring radionuclide or a naturally occurring inorganic substance.
As a result, the process for substances targeted for virtual elimination will be followed.
2. Background
2.1 Substance Information
PREPOD is part of the chemical grouping Amines and the chemical sub grouping Aromatic amines. PREPOD is the reaction product of N-phenyl-benzeneamine (diphenylamine or DPA) and 2-propanone (acetone). It is a UVCB (Unknown or Variable Composition, Complex Reaction Products, or Biological Materials) mixture, and, as such, contains a number of components in different concentrations.
Table 1a presents other names, trade names, chemical groupings, the chemical formula, the chemical structure and the molecular mass for PREPOD.
| Chemical Abstracts Service Registry Number (CAS RN) | 68412-48-6 |
|---|---|
| DSL name | 2-Propanone, reaction products with diphenylamine |
| National Chemical Inventories (NCI) names [1] | Reaction product from diphenylamine and acetone(ENCS) Condensate, acetone-diphenylamine (PICCS) Reaction product, diphenylamine-acetone (PICCS) Diphenylamine-acetone condensation product (PICCS) |
| Other names including tradenames | Acetone, diphenylamine condensation product Acetone diphenylamine condensation products[2] acetone; dicyclohexylamine[2] acetone; N-cyclohexylcyclohexanamine[2] ADPAL[3] BLE[4] CID162214[2] Diphenylamine, acetone reaction product EINECS 270-192-0[2] LS-123178[2] N-cyclohexylcyclohexanamine; propan-2-one[2] N-Phenylbenzeneamine, 2-propanone reaction product Rubber Antioxidant BLE[2] |
| Chemical group (DSL Stream) | Organic UVCB[5] |
| Major chemical class or use | Amines |
| Major chemical sub-class | Aromatic amines |
| Chemical formulae of reactants | C12H11N ; C3H6O |
| Structure of reactants | 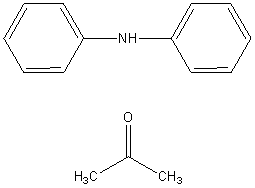 |
[2] ChemIndustry.com Inc. 2008.
[3] Chemicalland 2010.
[4] Chemtura Corporation 2007.
[5] This substance is a UVCB (Unknown or Variable Composition, Complex Reaction Products, or Biological Materials); i.e., it is not a discrete chemical and may be characterized by a variety of structures.
Table 1b shows the identity information (e.g., CAS RN) for the main components in PREPOD that were evaluated as representative for the purposes of the assessment. Not all components of PREPOD are shown. For more information, please refer to the final screening assessment report.
Table 1b. Identity and structure of the main components in PREPOD used in the assessment
| Chemical Abstracts Service Registry Number (CAS RN) | 122-39-4 |
|---|---|
| DSL name | Benzenamine, N-phenyl- |
| Common name | Diphenylamine (DPA) |
| Chemical formula | C12 H11 N |
| Structure (used to run the estimation models) |  |
| SMILES used to run the estimation models[2] | c1(Nc2ccccc2)ccccc1 |
| Molecular mass | 169.226 g/mol |
[2] Simplified Molecular Line Input Entry System.
| Chemical Abstracts Service Registry Number (CAS RN) | 6267-02-3 |
|---|---|
| DSL name | Acridine, 9,10-dihydro-9,9-dimethyl- |
| Common name | 9,9-dimethylacridan |
| Chemical formula | C15H15 N |
| Structure (used to run the estimation models) |  |
| SMILES used to run the estimation models[2] | c12C(c3c(cccc3)Nc1cccc2)(C)C |
| Molecular mass | 209.29 g/mol |
[2] Simplified Molecular Line Input Entry System.
| Chemical Abstracts Service Registry Number (CAS RN) | None |
|---|---|
| DSL name | Not on DSL |
| Common name | Diisopropyldimethylacridan |
| Chemical formula | C21H27N |
| Structure (used to run the estimation models) | 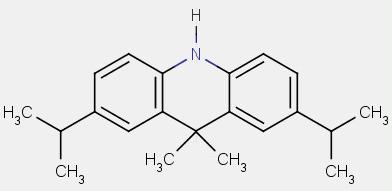 |
| SMILES used to run the estimation models[2] | N1c3ccc(cc3C(C)(C)c2c1ccc(c2)C(C)C)C(C)C |
| Molecular mass | 293.46 |
[2] Simplified Molecular Line Input Entry System.
| Chemical Abstracts Service Registry Number (CAS RN) | None |
|---|---|
| DSL name | Not on DSL |
| Common name | - |
| Chemical formula | C27 H26 N2 |
| Structure (used to run the estimation models) | 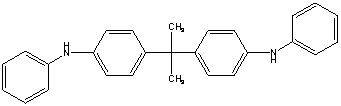 |
| SMILES used to run the estimation models[1] | c1ccccc1Nc2ccc(cc2)C(C)(C)c3ccc(cc3)Nc4ccccc4 |
| Molecular mass | 378.52 g/mol |
3. Why We Need Aaction
3.1 Characterization of Risk
Given the information on the amount of PREPOD that is manufactured, imported, and used in Canada, and on the nature of its reported uses, there is potential for release of PREPOD into the Canadian environment. Once released in the environment, because of its resistance to degradation, Components A, B, C, and D of PREPOD are expected to remain in water, sediment and soil for a long time. Because of the lipophilic character of Component C (Diisopropyldimethylacridan), and as it also persists in the environment, this component will likely bioaccumulate and may be biomagnified in trophic food chains. Modelled data suggest that Components C and D could potentially have high acute and chronic aquatic toxicity.
A site-specific risk quotient analysis, integrating estimates of exposure with toxicity information, was performed for the aquatic medium at three sites to determine whether there is potential for ecological harm in Canada. Predicted Environmental Concentrations (PECs) were estimated for all components of PREPOD at all three sites. Predicted No Effect Concentrations (PNECs) were derived from critical toxicity values (CTVs) chosen from modeled 30-day chronic values (ChV) for rainbow trout. As a result (refer to Table 2 below), one site was identified as having a risk quotient (RQ) above the level of concern (> 1); therefore, harm to aquatic organisms is possible at this site from the release of at least one of the component of PREPOD, Component C (Canada 2010).
| PREPOD Component | PEC (µg/L) | PNEC (µg/L) | RQ (PEC/PNEC) |
|---|---|---|---|
| Component A: site 1 (manufacture) | 2.43 | 72
| 0.034 |
| Component A: site 2 (use) | 0.063 | 0.0009 | |
| Component A: site 3 (use) | 0.535 | 0.007 | |
| Component B: site 1 (manufacture) | 1.03 | 26 | 0.04 |
| Component B: site 2 (use) | 0.026 | 0.001 | |
| Component B: site 3 (use) | 0.23 | 0.009 | |
| Component C: site 1 (manufacture) | 0.215 | 0.1 | 2.15 |
| Component C: site 2 (use) | 0.005 | 0.05 | |
| Component C: site 3 (use) | 0.0473 | 0.47 | |
| Component D: site 1 (manufacture) | 0.028 | 0.11 | 0.25 |
| Component D: site 2 | 0.0007 | 0.006 | |
| Component D: site 3 | 0.0062 | 0.056 |
Based on this information, PREPOD has the potential to cause ecological harm in Canada (Canada 2010).
4. Current Uses and Industrial Sectors
Information gathered from the CEPA 1999 Section 71 notices for the 2006 calendar year indicates that the total quantity of PREPOD that was manufactured in Canada was in the 100 000 to 1 000 000 kg/year range (Environment Canada 2006 and 2010).
For the 2006 calendar year, fewer than four Canadian companies reported importing PREPOD (as a component of vehicle parts and already assembled vehicles in the automobile industry) and the total quantity imported was in the 100 to 1 000 kg/year range (Environment Canada 2010).
According to submissions made under section 71 of CEPA, 1999, between 100 000 to 1 000 000 kg of PREPOD were used in 2006 (Environment Canada 2010).
The main reported use of PREPOD is as an antioxidant in rubber products. The industrial functions of PREPOD reported in the responses to the CEPA 1999 Section 71 notices for the 2005 and 2006 calendar years (Environment Canada 2006 and 2010) are: antioxidant, paint additive, coating additive, plasticizer, abrasives, oxidizing or reducing agent (Environment Canada 2010).
In addition, PREPOD is present in imported vehicle parts, namely in front mounting bracket for engines and in already assembled automobiles at concentrations of 0.0023% by weight and 0.0003% by weight, respectively (Environment Canada 2010).
The use codes for PREPOD, identified when the DSL was compiled in 1984-86, are shown below:
07- Antioxidant/corrosion inhibitor/tarnish inhibitor/scavenger/antiscaling agent
76- Organic Chemicals, Industrial
5. Presence in the Canadian Environment and Exposure Sources
5.1 Releases to the Environment
The losses estimated for PREPOD over its lifecycle are presented in Table 3 (Environment Canada 2010).
| Type of Loss | Proportion (%) | Pertinent Lifecycle Stages |
|---|---|---|
| Wastewater | 6.2 | Manufacture, industrial use, consumer/ commercial use |
| Land | - | – |
| Air | 0.1 | Manufacture, industrial use |
| Chemical transformation | Non-zero[2] | Industrial use, consumer/commercial use |
| Incineration | 3.3 | Industrial use, consumer/commercial use |
| Landfill | 82.9 | Manufacture, industrial use, consumer/commercial use |
| Recycling | 7.6 | – |
| Export | 0 | – |
[2] Potential chemical transformation of PREPOD stemming from the process of oxidation is acknowledged; however, at the present time the extent to which it occurs is not adequately documented in the available literature to allow quantification.
Industrial Releases
Potential releases from PREPOD manufacturing were estimated to be about 6.2% to water. Environmental concentrations were estimated and used to predict site-specific exposure from the potential releases of PREPOD as a result of its use in its formulation and industrial use as a rubber additive. One site was identified as having the potential to cause ecological harm from the release of at least one of the component of PREPOD, Component C.
Product Releases
The above loss estimates indicate that PREPOD has a potential for release to the environment. Due to the low quantity of PREPOD used in finished vehicles and car parts, its function, and the anticipated recycling or incineration of many of these vehicle parts, significant losses are not expected from these sources.
In general, wastewater is a common source for releases of a substance to water and soil through wastewater treatment facilities and the subsequent waste management of sludge.
Landfills are where the majority of the substance is expected to end up. This substance has the potential to leach the substance into groundwater. In areas where landfill leachate is collected and sent to a local sewage treatment plant for treatment, the substance can enter the receiving water via the effluent as well as the soil when biosolids from the plant are applied to it.
5.2 Exposure Sources
PREPOD is not naturally produced in the environment. Empirical data on concentrations of PREPOD in any environmental media in Canada were not identified.
Given the information on the amount of PREPOD that is imported and manufactured in Canada and on the nature of its reported uses, there is potential for releases of PREPOD to the Canadian environment. Once released in the environment, because of its resistance to degradation Components A, B, C, and D of PREPOD are expected to remain in water, sediment and soil for a long time. Because of the lipophilic character of Component C (Diisopropyldimethylacridan), and as it also persists in the environment, this component will likely bioaccumulate and may be biomagnified in trophic food chains. Modelled data suggest that Components C and D could potentially have high acute and chronic aquatic toxicity. Based on this information, PREPOD has the potential to cause ecological harm in Canada.
General population exposure to PREPOD from use of consumer products is not anticipated. Exposure of the general population to PREPOD through environmental media (air, drinking water and soil) and food and beverages is expected to be negligible.
6. Overview of Existing Actions
6.1 Existing Canadian Risk Management
No existing Canadian risk management was identified.
6.2 Existing International Risk Management
- United States of America: the substance is on the Toxic Substances Control Act Chemical Substance Inventory (US EPA 1985).
- European Union: the substance is on the European Inventory of Existing Commercial chemical Substances (EINECS) inventory (ESIS 1995-2010).
- Japan: PREPOD is on the list of Existing substances of the Chemical Substances Control Law (CHRIP 2008).
- International Maritime Organization (UN): PREPOD is featured in the Dangerous Goods List of the IMDG Code (the International Maritime Dangerous Goods Code) where it is classified as an environmentally hazardous substance, solid, not otherwise specified and as a marine pollutant.
- New Zealand: 2-Propanone, reaction products with diphenylamine (CAS RN 68412-48-6) may be used as a component in a product covered by a group standard but it is not approved for use as a chemical in its own right (Added: 1/12/2006) (New Zealand Inventory of Chemicals).
7. Considerations
7.1 Alternative Chemicals or Substitutes
At this time, no suitable alternative chemicals, or substitutes have been identified.
7.2 Alternative Technologies and/or Techniques
At this time, no alternative technologies and/or alternative techniques that would minimize or eliminate the use and/or release of the substance have been identified.
7.3 Socio-economic Considerations
Socio-economic factors have been considered in the selection process for a regulation and/or instrument respecting preventive or control actions, and in the development of the risk management objective(s). Socio-economic factors will also be considered in the development of regulations, instrument(s) and/or tool(s) as identified in the Cabinet Directive on Streamlining Regulation[3] (Treasury Board of Canada Secretariat 2007) and the guidance provided in the Treasury Board document Assessing, Selecting, and Implementing Instruments for Government Action .
Socio-economic considerations for PREPOD include:
- Costs and benefits for the Canadian government, industry and consumers.
8. Proposed Objectives
8.1 Environmental Objective
An environmental objective is a quantitative or qualitative statement of what should be achieved to address environmental concerns identified during a risk assessment.
The ultimate environmental objective for PREPOD is virtual elimination (VE) of releases into the environment. CEPA 1999 requires that substances targeted for VE under section 77 be added to the Virtual Elimination List. According to CEPA 1999, virtual elimination means, in respect of a toxic substance released into the environment as a result of human activity, the ultimate reduction of the quantity or concentration of the substance in the release.
8.2 Risk Management Objective
A risk management objective is a target expected to be achieved for a given substance by the implementation of risk management regulations, instrument(s) and/or tool(s).
The proposed risk management objective for PREPOD is to minimize releases of the substance from the manufacture of the substance to the greatest extent possible.
9. Proposed Risk Management
9.1 Proposed Risk Management Instrument and Tool
As required by the Government of Canada’s Cabinet Directive on Streamlining Regulation and criteria identified in the Treasury Boarddocument entitled Assessing, Selecting, and Implementing Instruments for Government Action, the proposed risk management was selected using a consistent approach, and took into consideration the information that was received through the Challenge and other information available at the time.
In order to achieve the risk management objective and to work towards achieving the environmental objective, the Government of Canada is proposing the following actions for PREPOD:
- Adding PREPOD to the Virtual Elimination List of CEPA 1999.
- Addressing releases from the manufacturing and industrial use of PREPOD by developing a control instrument under CEPA 1999, as appropriate.
- Implementing Significant New Activity (SNAc) provisions under CEPA 1999 for PREPOD.
- Working with stakeholders to further quantify sources of releases of PREPOD to the environment throughout its lifecycle and developing risk management control actions under CEPA 1999 to address these releases as required.
- Adding PREPOD to the CMP monitoring program to quantify levels of this substance that may be found in the environment.
9.2 Implementation Plan
The proposed measures respecting preventative or control actions in relation to PREPOD will be published in the Canada Gazette, Part I, by September 2013, as per the timelines legislated in CEPA 1999.
10. Consultation Approach
The risk management scope document for PREPOD, which summarized the proposed risk management under consideration at that time, was published on October 2, 2010. Industry and other interested stakeholders were invited to submit comments on the risk management scope document during a 60-day comment period. Comments received on the risk management scope document were taken into consideration in the development of this proposed risk management approach document.
The primary stakeholders include:
- Chemical manufacturers, importers and distributors
- Rubber manufacturers, importers and distributors
- Automotive sector
- Non-governmental organizations
- Provincial/territorial gouvernements
There will be additional opportunities for public consultation during the development of the risk management instrument.
11. Next Steps / Proposed Timeline
| Actions | Date |
|---|---|
| Electronic consultation on proposed risk management approach document | September 10, 2011 to November 9, 2011 |
| Response to comments on proposed the risk management approach document | No later than the time of publication of the proposed instrument |
| Consultation on the draft instrument | Spring/Summer 2012 |
| Publication of the proposed instrument | No later than September 2013 |
| Formal public comment period on the proposed instrument | No later than Fall 2013 |
| Publication of the final instrument | No later than March 2015 |
Industry and other interested stakeholders are invited to submit comments on the content of this proposed risk management approach or provide other information that would help to inform decision making. Please submit comments prior to November 9, 2011 since the risk management of PREPOD will be moving forward after this date. During the development of regulations, instrument(s) and tool(s), there will be opportunity for consultation. Comments and information submissions on the proposed risk management approach should be submitted to the address provided below:
Chemicals Management Division
Gatineau Quebec K1A 0H3
Tel: 1-888-228-0530 / 819-956-9313
Fax: 819-953-7155
Email: Substances@ec.gc.ca
12. References
Canada. 1999. Canadian Environmental Protection Act, 1999. S.C. 1999, Ch. 33. Canada Gazette, Part III. Vol. 22, No. 3.
Canada. 2000. Canadian Environmental Protection Act, 1999: Persistence and Bioaccumulation Regulations, P.C. 2000-348, 23 March 2000, SOR/2000-107. Canada Gazette, Part II, Vol. 134, No. 7.
Canada. 2009. Dept. of the Environment. Canadian Environmental Protection Act, 1999: Notice with respect to Batch 11 Challenge substances. Canada Gazette, Part I, Vol. 143, No. 39.
Canada. 2010. Environment Canada, Health Canada. Final Screening Assessment for the Challenge, 2-Propanone, reaction products with diphenylamine (PREPOD), Chemical Abstract Service Registry Number (CAS RN) 68412-48-6. Chemical Substances in Batch 11 of the Challenge.
Chemicalland. 2010. Fact Sheet: Acetone Diphenylamine.
ChemIndustry.com Inc 2008. Chemical Information Seach for Acetone Dicyclohexylamine.
[CHRIP] Chemical Risk Information Platform [database on the Internet]. c2008. Tokyo (JP): National Institute of Technology and Evaluation, Chemical Management Centre (CMC). [cited 2010 5 14].
CRA [Conestoga-Rovers & Associates]. 2010. Air Quality and Deposition Assessment BLE 25® Emergency Vent Release. Prepared for Chemtura Co., Elmira, Ontario.
Environment Canada. 1988. Data relating to the Domestic Substance List (DSL) 1984-1986, collected under CEPA, 1988, s. 25(1). Collected according to: Reporting for the Domestic Substance List [reporting guide], Minister of Supply and Services Canada, DSS cat. no. En40-364/1998E. Prepared by: Environment Canada, New Substances Division.
Environment Canada. 2006. Data for selected substances collected under the Canadian Environmental Protection Act, 1999, Section 71: Notice with respect to selected substances identified as priority for action. Prepared by: Environment Canada, Health Canada, Existing Substances Program.
Environment Canada. 2010. Data for Batch 11 substances collected under the Canadian Environmental Protection Act, 1999, Section 71: Notice with respect to certain Batch 11 Challenge substances. Data compiled by: Environment Canada, Program Development and Engagement Division.
[ESIS] European Chemical Substances Information System [European Inventory of Existing Commercial chemical Substances database on the Internet]. c1995-2010. European Chemical Bureau (ECB). [cited 2010 August].
[NCI] National Chemical Inventories [database on CD-ROM]. 2009. Issue 1. Columbus (OH): American Chemical Society. [cited 2010 5 14].
New Zealand Inventory of Chemicals [database on the Internet]. Environmental Risk Management Authority.
Treasury Board of Canada Secretariat. 2007. Cabinet Directive on Streamlining Regulation, section 4.4.
US EPA. 1985. Toxic Substances Control Act (TSCA) Chemical Substance Inventory: TSCA Inventory: 1985 Edition, Volume II: User Guide and Indices to the TSCA Inventory, Substance Name Index. TSCA Chemical Substance Inventory.
Footnotes
[2] CAS RN: Chemical Abstracts Service Registry Number. The Chemical Abstracts Service information is the property of the American Chemical Society and any use or redistribution, except as required in supporting regulatory requirements and/or for reports to the Government of Canada when the information and the reports are required by law or administrative policy, is not permitted without the prior, written permission of the American Chemical Society.
[3] Section 4.4 of the Cabinet Directive on Streamlining Regulation states that “Departments and agencies are to: identify the appropriate instrument or mix of instruments, including regulatory and non-regulatory measures, and justify their application before submitting a regulatory proposal”.
[*] Note: This summary is an abridged list of the instruments and tools proposed to risk manage this substance. Please see section 9.1 of this document for a complete explanation of risk management.
- Date modified: